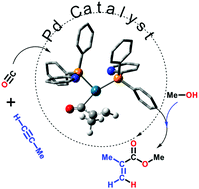
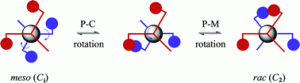
Palladium-catalysed alkyne alkoxycarbonylation with P,N-chelating ligands revisted: a density functional theory study
Shabhaz Ahmad, Ashley Lockett, Timonthy A. Shuttleworth, Alexandra M. Miles-Hobbs, Paul G. Pringle and Michael Bühl
Phys. Chem. Chem. Phys., 2019, Advanced Article
Phosphophosphidites Derived from BINOL
Adam. D. Gorman, Jessica A. Cross, Rachel A. Doyle, Tom R. Leonard, Paul G. Pringle, Hazel A. Sparkes

Eur. J. Inorg. Chem., 2019, 1633-1639.
Inorganic Triphenylphosphine
Adam D. Gorman, Jonathan A. Bailey, Natalie Fey, Tom A. Young, Hazel A. Sparkes, Paul G. Pringle
Angew. Chem. Int. Ed., 2018, 57, 15802-15806.
Pyrrolidines and Piperidines by Ligand-Enabled Aza-Heck Cylizations and Cascades of N-(Pentafluorobenzoyloxy)carbamates
Ian R. Hazelden, Rafaela C. Carmona, Thomas Langer, Paul G. Pringle and John F. Bower
Angew. Chem. Int. Ed., 2018, 57, 5124-5128.
Spin changes accompany ultrafast structural interconversion in the ground state of a colbalt nitrosyl complex
Hugo J. B. Marroux, Basile F. E. Curchod, Charly A. Faradji, Timothy A. Shuttleworth, Hazel A. Sparkes, Paul G. Pringle and Andrew J. Orr-Ewing
Angew. Chem. Int. Ed., 2017, 56, 13713-13716.
2-Pyridyl substituents enhance the activity of palladium-phospha-adamantane catalysts for the methoxycarbonylation of phenylacetylene
Timothy A. Shuttleworth, Alexandra M. Miles-Hobbs, Paul G. Pringle and Hazel A. Sparkes
Dalton Trans., 2017, 46, 125-137.
David J. Lunn, Oliver E. C. Gould, George R. Whittell, Daniel P. Armstrong, Kenneth P. Mineart, Mitchell A. Winnik, Richard J. Spontak, Paul G. Pringle and Ian Manners.
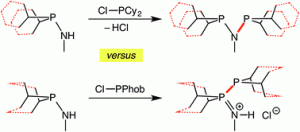
Aminophobanes: hydrolytic stability, tautomerism and application in Cr-catalysed ethene oligomerisation
Mairi F. Haddow, Judit Jaltai, Martin Hanton, Paul G. Pringle Laura E. Rush, Hazel A. Sparkes, Christopher H. Woodall
Dalton Trans., 2016, 45, 2294-2307.
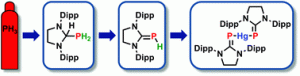
Carbene insertion into a P-H bond: parent phosphinidene-carbene adducts from PH3 and bis(phosphinidene) mercury complexes
Mark Bispinghoff, Aaron M. Tondreau, Hansjörg Grützmacher, Charly A. Faradji, Paul G. Pringle
Dalton Trans., 2016, advance article
Subtle effects of ligand backbone on the efficiency of iron-diphos catalysed Negishi cross-coupling reactions
Jamie Clifton, Evi R. M. Habraken, Ian Manners, Paul G. Pringle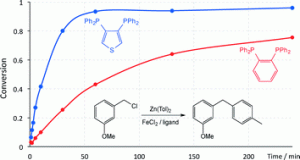
Catal, Sci. Technol. 2015, 5, 4350-4353.
One-step Modular route to optically active diphos ligands
E. Louise Hazeland, Andy M. Chapman, Paul G. Pringle,
Hazel A. Sparkes
 Chem. Commun, 2015, 10206-10209.
Chem. Commun, 2015, 10206-10209.
Monomeric Phosphinoboranes

Jonathan A. Bailey, Paul G. Pringle
Coord. Chem. Rev, 2015, 297-298, 77-90.
Single Oxygen-Atom Insertion into P-B Bonds: On- and Off-Metal Transformation of a Borylphosphine into a Borylphosphinite
 Jonathan A. Bailey, Hazel A. Sparkes, Paul G. Pringle
Jonathan A. Bailey, Hazel A. Sparkes, Paul G. Pringle
Chem. Eur. J., 2015, 21, 5360-5363.
Cooperative Lewis Pairs Based on Late Transition Metals: Activation of Small Molecules by Platinum(0) and B(C6F5)3
Sebastian J. K. Forrest, Jamie Clifton, Natalie Fey, Paul G. Pringle, Hazel A. Sparkes, Duncan F. Wass
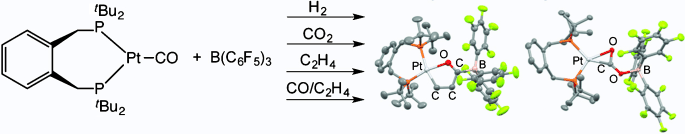
Angew. Chem. Int. Ed,, 2015, 54, 2223-2227
Setting P-Donor Ligands into Context: An Application of the Ligand Knowledge Base (LKB) Approach
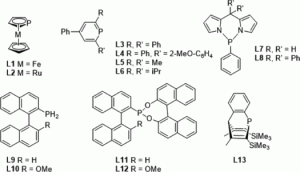
Natalie Fey, Sofia Papadouli, Paul G. Pringle, Arne Ficks, James T. Fleming, Lee J. Higham, Jennifer F. Wallis, Duncan Carmichael, Nicolas Mézailles, Christian Müller
Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 706-714.
Reversible CO Exchange at Platinum(0). An Example of Similar Complex Properties Produced by Ligands with Very Different Stereoelectronic Characteristics
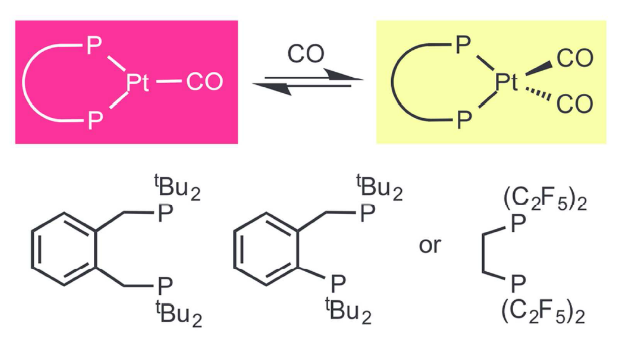
Paul G Pringle, Sebastian Forrest, Hazel Sparkes, Duncan Wass
Dalton Trans., 2014, 43, 16335-16344.
Computational Kinetics of Cobalt-Catalyzed Alkene Hydroformylation
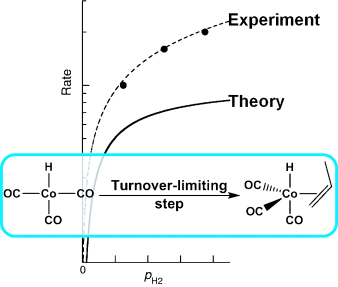
Laura E. Rush, Paul G. Pringle, Jeremy N. Harvey
Angew. Chem. Int. Ed. 2014, 53, 8672–8676.
Mono-, Di-, and Triborylphosphine Analogues of Triarylphosphines
Jonathan A. Bailey, Marten Ploeger, Paul G. Pringle
Inorg. Chem., 2014, 53, 7763–7769.
Cobalt PCP Pincer Complexes via an Unexpected Sequence of Ortho Metalations
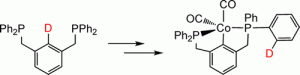
Mark A. Kent , Christopher H. Woodall, Mairi F. Haddow, Claire L. McMullin, Paul G. Pringle, Duncan F. Wass
Organometallics, 2014, 33, 5686-5692.
Correlations of the Structural Properties of a Complete R2PX Series (X = Hydrogen or Halogen)
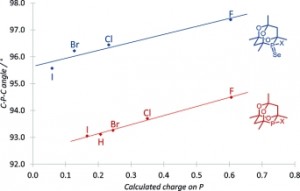
Jonathan P. Hopewell, Claire L. McMullin, Paul G. Pringle, Timothy A. Shuttleworth, Christopher H. Woodall
Eur. J. Inorg. Chem., 2014, 1843–1849.
Unexpectedly High Barriers to M-P Rotation in Tertiary Phobane Complexes: PhobPR Behavior That Is Commensurate with tBu2PR
Julia M. Lister, Monica Carreira, Mairi F. Haddow, Alex Hamilton, Claire L. McMullin, A. Guy Orpen, Paul G. Pringle, Tom E. Stennett
Organometallics, 2014, 33, 702–714
A simple route to azaborinylphosphines: isoelectronic B–N analogues of arylphosphine ligands
 Jonathan A. Bailey, Mairi F. Haddow, Paul G. Pringle
Jonathan A. Bailey, Mairi F. Haddow, Paul G. Pringle
Chem. Commun., 2014, 50, 1432-1434
Interplay of bite angle and cone angle effects. A comparison between o-C6H4(PR2)(CH2PR2‘) and o-C6H4(CH2PR2)(CH2PR2‘) as ligands for Pd-catalysed ethene hydromethoxycarbonylation.
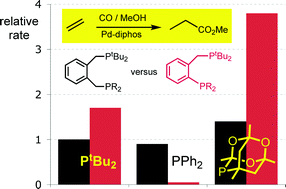 T. Fanjul, G. Eastham, J. Floure, S. J. K. Forrest, M. F. Haddow, A. Hamilton, P. G. Pringle, A. G. Orpen, M. Waugh
T. Fanjul, G. Eastham, J. Floure, S. J. K. Forrest, M. F. Haddow, A. Hamilton, P. G. Pringle, A. G. Orpen, M. Waugh
Dalton Trans., 2013, 42, 100-115.
Stable Fluorophosphines: Predicted and Realized Ligands for Catalysis.
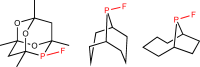 N. Fey, M. Garland, J. P. Hopewell, C. L. McMullin, S. Mastroianni, A. G. Orpen, P. G. Pringle,
N. Fey, M. Garland, J. P. Hopewell, C. L. McMullin, S. Mastroianni, A. G. Orpen, P. G. Pringle,
Angew. Chem. Int. Ed., 2012, 51, 118-122
Regioselective B-Cyclometalation of a Bulky o-Carboranyl Phosphine and the Unexpected Formation of a Dirhodium(II) Complex.
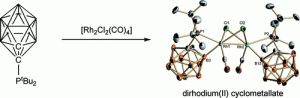 N. Fey, M. F. Haddow, R. Mistry, N. C. Norman, A. G. Orpen, T. J. Reynolds, P. G. Pringle
N. Fey, M. F. Haddow, R. Mistry, N. C. Norman, A. G. Orpen, T. J. Reynolds, P. G. Pringle
Organometallics, 2012, 31, 2907–2913.
Efficient and chemoselective ethene hydromethoxycarbonylation catalysts based on Pd-complexes of heterodiphosphines o-C6H4(CH2PtBu2)(CH2PR2).
 T. Fanjul, G. Eastham, M. F. Haddow, A. Hamilton, P. G. Pringle, A. G. Orpen, T. P. W. Turner, M. Waugh
T. Fanjul, G. Eastham, M. F. Haddow, A. Hamilton, P. G. Pringle, A. G. Orpen, T. P. W. Turner, M. Waugh
Catal. Sci. Technol., 2012, 2, 937-950.
Diphosphanes derived from phobane and phosphatrioxa-adamantane: similarities, differences and anomalies.
 D. L. Dodds, J. Floure, M. Garland, M. F. Haddow, T. R. Leonard, C. L. McMullin, A. G. Orpen, P. G. Pringle
D. L. Dodds, J. Floure, M. Garland, M. F. Haddow, T. R. Leonard, C. L. McMullin, A. G. Orpen, P. G. Pringle
Dalton Trans., 2011, 40, 7137-7146.
Cage Phosphinites: Ligands for Efficient Nickel-Catalyzed Hydrocyanation of 3-Pentenenitrile.
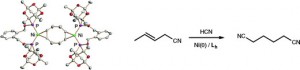 I. S. Mikhel, M. Garland, J. Hopewell, S. Mastroianni, C. L. McMullin, A. G. Orpen, P. G. Pringle
I. S. Mikhel, M. Garland, J. Hopewell, S. Mastroianni, C. L. McMullin, A. G. Orpen, P. G. Pringle
Organometallics 2011, 30, 974–985.
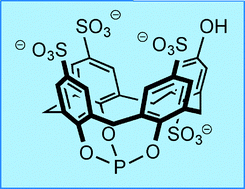
A water-soluble phosphite derived from sulfonated calix[4]arene. The remarkable stability of its rhodium complexes and two phase hydroformylation studies.
Catal. Sci. Technol., 2011, 1, 239-242.
Is restricted M–P rotation a common feature of enantioselective monophos catalysts? An example of restricted Rh–P rotation in a secondary phosphine complex.
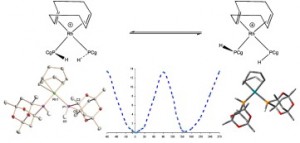 P. Jankowski, C. L. McMullin, I. D. Gridnev, A. G. Orpen, P. G. Pringle
P. Jankowski, C. L. McMullin, I. D. Gridnev, A. G. Orpen, P. G. Pringle
Tetrahedron Asym., 2010, 21, 1206-1209.
Palladium Complexes of the Heterodiphosphine o-C6H4(CH2PtBu2)(CH2PPh2) Are Highly Selective and Robust Catalysts for the Hydromethoxycarbonylation of Ethene.
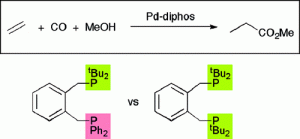 T. Fanjul , G. Eastham, N. Fey, A. Hamilton, P. G. Pringle, M. Waugh
T. Fanjul , G. Eastham, N. Fey, A. Hamilton, P. G. Pringle, M. Waugh
Organometallics, 2010, 29, 2292-2305.
Subtleties in asymmetric catalyst structure: the resolution of a 6-phospha-2,4,8-trioxa-adamantane and its applications in asymmetric hydrogenation catalysis.
 J. Hopewell, P. Jankowski, C. L. McMullin, A. G. Orpen, P. G. Pringle
J. Hopewell, P. Jankowski, C. L. McMullin, A. G. Orpen, P. G. Pringle
Chem Commun., 2010, 46, 100-102.
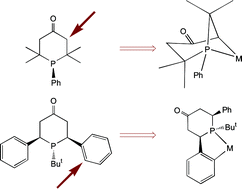
Anatomy of Phobanes. Diastereoselective Synthesis of the Three Isomers of n-Butylphobane and a Comparison of their Donor Properties.
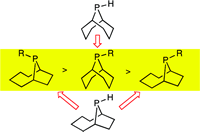 M. Carreira, M. Charernsuk, M. Eberhard, N. Fey, R. van Ginkel, A. Hamilton, W. P. Mul, A. G. Orpen, H. Phetmung, P. G. Pringle
M. Carreira, M. Charernsuk, M. Eberhard, N. Fey, R. van Ginkel, A. Hamilton, W. P. Mul, A. G. Orpen, H. Phetmung, P. G. Pringle
J. Am. Chem. Soc , 2009, 131, 3078-3092.
Stereoelectronic effects in a homologous series of bidentate cyclic phosphines. A clear correlation of hydroformylation catalyst activity with ring size.
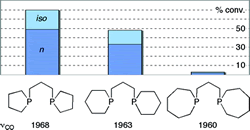 M. F. Haddow, A. J. Middleton, A. G. Orpen, P. G. Pringle, R. Papp
M. F. Haddow, A. J. Middleton, A. G. Orpen, P. G. Pringle, R. Papp
Oxidative Dehydrogenation of Tris(o-isopropylphenyl)phosphines by Platinum Complexes.
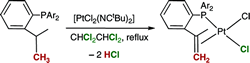 A. Baber, C. Fan, D. W. Norman, A. G. Orpen, P. G. Pringle, R. L. Wingad
A. Baber, C. Fan, D. W. Norman, A. G. Orpen, P. G. Pringle, R. L. Wingad
Organometallics, 2008, 27, 5906-5910.
General Routes to Alkyl Phosphatrioxaadamantane Ligands.
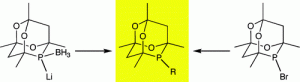 J. H. Downing, J. Floure, K. Heslop, M. F. Haddow, J. Hopewell, M. Lusi, H. Phetmung, A. G. Orpen, P. G. Pringle, R. I. Pugh, D. Zambrano-Williams
J. H. Downing, J. Floure, K. Heslop, M. F. Haddow, J. Hopewell, M. Lusi, H. Phetmung, A. G. Orpen, P. G. Pringle, R. I. Pugh, D. Zambrano-Williams
Organometallics, 2008, 27, 3216–3224.
Bidentates versus Monodentates in Asymmetric Hydrogenation Catalysis: Synergic Effects on Rate and Allosteric Effects on Enantioselectivity.
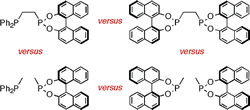 D. W. Norman, C. A. Carraz, D. J. Hyett, P. G. Pringle, J. B. Sweeney, A. G. Orpen, H. Phetmung, R. L. Wingad
D. W. Norman, C. A. Carraz, D. J. Hyett, P. G. Pringle, J. B. Sweeney, A. G. Orpen, H. Phetmung, R. L. Wingad
J. Am. Chem. Soc. 2008, 130, 6840-6847.
A simple entry into nido-C2B10 clusters: HCl promoted cleavage of the C–C bond in ortho-carboranyl diphosphines.
 J. P. H. Charmant, M. F. Haddow, R. Mistry, N. C. Norman, A. G. Orpen, P. G. Pringle
J. P. H. Charmant, M. F. Haddow, R. Mistry, N. C. Norman, A. G. Orpen, P. G. Pringle
Dalton Trans, 2008, 1409-1411.
New insights into the mechanism of asymmetric hydrogenation catalysed by monophosphonite-rhodium complexes.
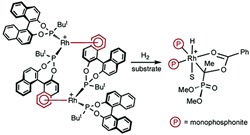 I. D. Gridnev, C. Fan, P. G. Pringle
I. D. Gridnev, C. Fan, P. G. Pringle
Chem. Commun., 2007, 1319-1321.
Ligand stereoelectronic effects in complexes of phospholanes, phosphinanes, and phosphepanes and their implications for hydroformylation catalysis.
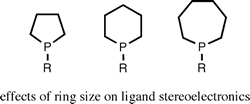 R. A. Baber, M. F. Haddow, A. J. Middleton, A. G. Orpen, P. G. Pringle, A. Haynes, G. L. Williams, R. Papp
R. A. Baber, M. F. Haddow, A. J. Middleton, A. G. Orpen, P. G. Pringle, A. Haynes, G. L. Williams, R. Papp
Organometallics, 2007, 26, 713-725.
Synthesis and reactivity of dichloroboryl complexes of platinum(II).
 J. P. H. Charmant, C. Fan, N. C. Norman, P. G. Pringle
J. P. H. Charmant, C. Fan, N. C. Norman, P. G. Pringle
Stereospecific diphosphination of activated acetylenes: a general route to backbone-functionalized, chelating 1,2-diphosphinoethenes.
![]() D. L. Dodds, M. F. Haddow, A. G. Orpen, P. G. Pringle, G. Woodward,
D. L. Dodds, M. F. Haddow, A. G. Orpen, P. G. Pringle, G. Woodward,
Organometallics, 2006, 25, 5937-5945.
Nitrogen-linked diphosphine ligands with ethers attached to nitrogen for chromium-catalyzed ethylene tri- and tetramerizations.
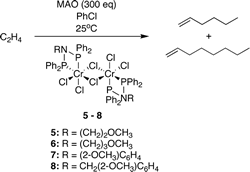 P. R. Elowe, C. McCann, P. G. Pringle, S. K. Spitzmesser, J. E. Bercaw
P. R. Elowe, C. McCann, P. G. Pringle, S. K. Spitzmesser, J. E. Bercaw
Organometallics, 2006, 25, 5255-5260.
Allosteric effects in asymmetric hydrogenation catalysis? Asymmetric induction as a function of the substrate and the backbone flexibility of C1-symmetric diphosphines in rhodium-catalysed hydrogenations.
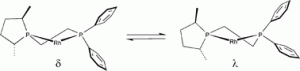 R. A. Baber, J. G. de Vries, A. G. Orpen, P. G. Pringle, K. von der Luehe
R. A. Baber, J. G. de Vries, A. G. Orpen, P. G. Pringle, K. von der Luehe
Dalton Trans., 2006, 4821-4828.
Chiral palladium bis(phosphite) PCP-pincer complexes via ligand C–H activation.
 R. A. Baber, R. B. Bedford, M. Betham, M. E. Blake, S. J. Coles, M.F. Haddow, M. B. Hursthouse, A. G. Orpen, L. T. Pilarski, P. G. Pringle, R. L. Wingad
R. A. Baber, R. B. Bedford, M. Betham, M. E. Blake, S. J. Coles, M.F. Haddow, M. B. Hursthouse, A. G. Orpen, L. T. Pilarski, P. G. Pringle, R. L. Wingad
Chem. Commun., 2006, 3880-3882.
Bulky 4-phosphacyclohexanones: diastereoselective complexations, orthometallations and unprecedented [3.1.1]metallabicycles.
R. Doherty, M. F. Haddow, Z. A. Harrison, A. G. Orpen, P. G. Pringle, A. Turner, R. L. Wingad
Dalton Trans., 2006, 4310-4320.
Ketone H2-hydrogenation catalysts: ruthenium complexes with the headphone-like ligand bis(phospha-adamantyl)propane.
 A. Hadovic, A. J. Lough, R. H. Morris, P. G. Pringle, D. E. Zambrano-Williams
A. Hadovic, A. J. Lough, R. H. Morris, P. G. Pringle, D. E. Zambrano-Williams
Inorg. Chim. Acta, 2006, 359, 2864-2869.
Nine-membered trans square-planar chelates formed by a bisbi analogue.
 M. R. Eberhard, K. M. Heslop, A. G. Orpen, P. G. Pringle
M. R. Eberhard, K. M. Heslop, A. G. Orpen, P. G. Pringle
Organometallics, 2005, 24, 335-337.
Separation of phobane isomers by selective protonation.
 M. R. Eberhard, E. Carrington-Smith, E. E. Drent, P. S. Marsh, A. G. Orpen, H. Phetmung, P. G. Pringle
M. R. Eberhard, E. Carrington-Smith, E. E. Drent, P. S. Marsh, A. G. Orpen, H. Phetmung, P. G. Pringle
Adv. Synth. Catal., 2005, 347, 1345-1348.
Special effects of ortho-isopropylphenyl groups. Diastereoisomerism in platinum(II) and palladium(II) complexes of helically chiral PAr3 ligands.
 R. A. Baber, A. G. Orpen, P. G. Pringle, M. J. Wilkinson, R. L. Wingad
R. A. Baber, A. G. Orpen, P. G. Pringle, M. J. Wilkinson, R. L. Wingad
Phenylphosphatrioxa-adamantanes: bulky, robust, electron-poor ligands that give very efficient rhodium(I) hydroformylation catalysts.
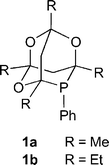 R. A. Baber, M. L. Clarke, K. M. Heslop, A. C. Marr, A. G. Orpen, P. G. Pringle, A. Ward, D. E. Zambrano-Williams
R. A. Baber, M. L. Clarke, K. M. Heslop, A. C. Marr, A. G. Orpen, P. G. Pringle, A. Ward, D. E. Zambrano-Williams
Dalton Trans., 2005, 1079-1085.
The electron-poor phosphines P{C6H3(CF3)2-3,5}3 and P(C6F5)3 do not mimic phosphites as ligands for hydroformylation. A comparison of the coordination chemistry of P{C6H3(CF3)2-3,5}3 and P(C6F5)3 and the unexpectedly low hydroformylation activity of their rhodium complexes.
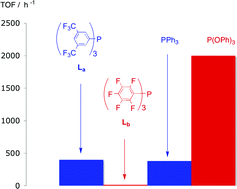 M. L. Clarke , D. Ellis, K. L. Mason, A. G. Orpen, P. G. Pringle, R. L. Wingad, D. A. Zaher, R. T. Baker
M. L. Clarke , D. Ellis, K. L. Mason, A. G. Orpen, P. G. Pringle, R. L. Wingad, D. A. Zaher, R. T. Baker
Dalton Trans., 2005,1294-1300.
Bulky triarylarsines are effective ligands for palladium catalysed Heck olefination.
 R. A. Baber, S. Collard, M. Hooper, A. G. Orpen, P. G. Pringle, M. J. Wilkinson, R. L. Wingad
R. A. Baber, S. Collard, M. Hooper, A. G. Orpen, P. G. Pringle, M. J. Wilkinson, R. L. Wingad